Which of the Following Molecules Contain the Same Functional Groups
CHCHNH2CH3 CHCH2CH2NH2 CH3CH2CONH2 CHCH2NHCH3 I II III IV A I П IV B I І Ш С І Ш V D I II IV 6. Which of the following molecules contain the same functional groups.

Functional Groups Introduction To Chemistry
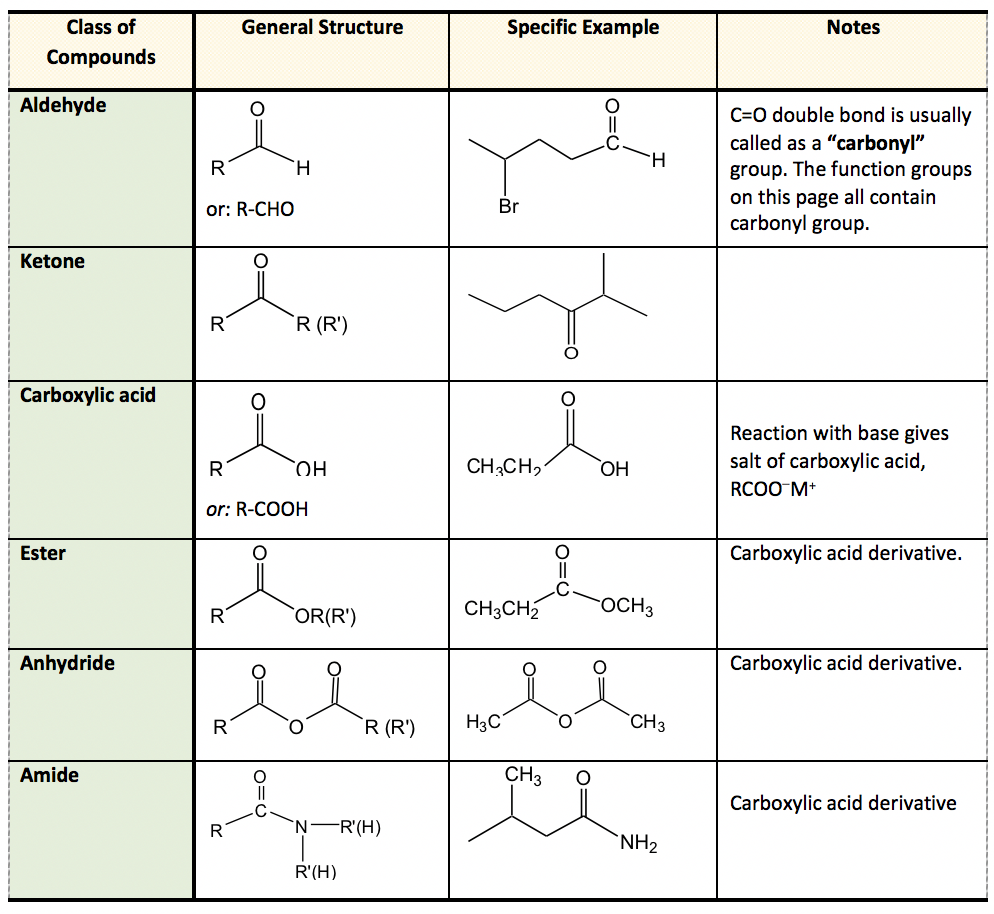
The carboxyl group contains the CO.

. Ketones contain a carbonyl group on a carbon within the chain c. Which of the following molecules contain the same functional groups. D Amine aromatic carboxylic acid ester nitrile.
CO stretching of carboxylic acid group 1700 to 1725 cm-1. Ketones are cyclic compounds. CO stretching of ketone group 1705 to 1725 cm-1.
These moieties the part of the molecule which can be found in many other molecules as well are responsible for the chemical reactions that the molecule they are attached to participate in. Carbonyl compounds show strong absorption at 1665 to 1830 cm-1. Which of the following correctly matches the molecules to the names of the functional groups.
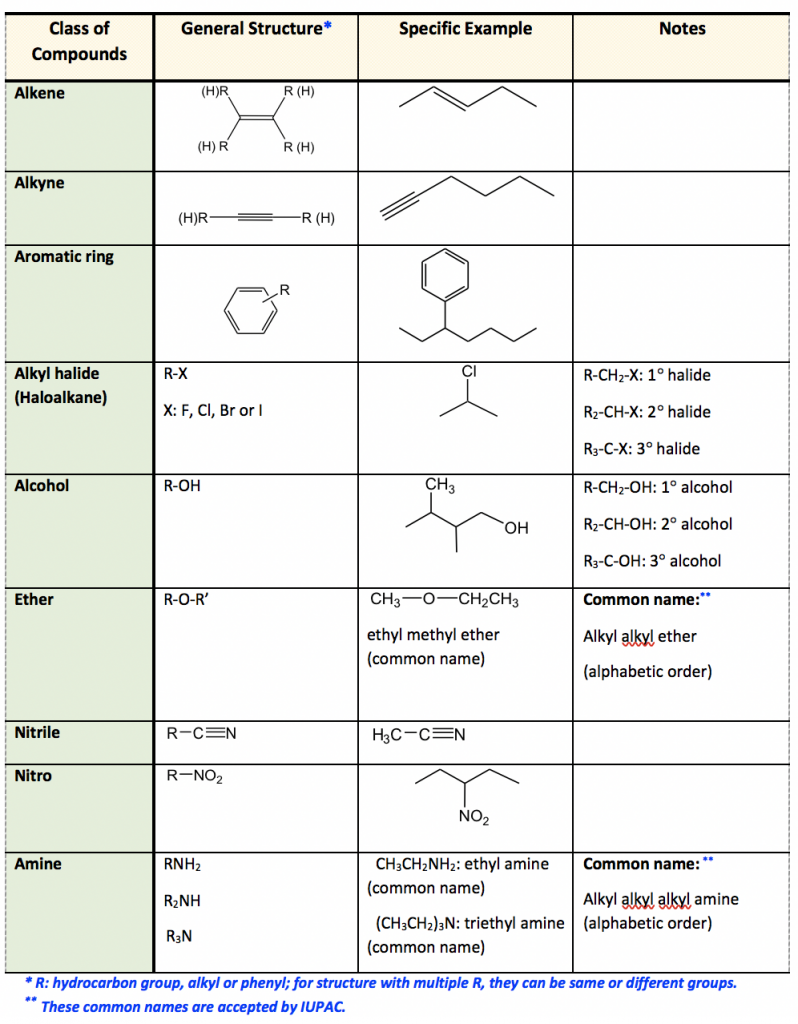
The carbonyl group of a ketone is bonded to an oxygen atom. CAlZ C 2 - CH1 2 CH2 - CH 2 CH2 - CHZY C12 - cutz 412 - CH2 CH 2 Cyclopropane cyclobutane cydopantare Cydohaxane 60 Amine. CH3CH3 CH3CH2OH CH2CH2 CH3OCH3 I II II IV A І II II В Iand II С II I IV D II and IV.
C Amide alcohol aromatic carboxylic acid ether. The functional group present in the following molecules is Carboxylic acid. The CO group is called a carbonyl carbon-EEL.
Organic acids such as acetic acid all contain a functional group called a carboxyl group. Draw simple organic molecule that contain following functional group - cycloalkane. Aldehydes contain a carbonyl group on an end carbon of the carbon chain.
Solve any question of Aldehydes Ketones and Carboxylic Acids with-. Which of the following molecules contain the same functional groups. To organic molecules functional groups which of the following lists contains common heteroatoms found in organic molecules.
The first sugar has an OH group on both the first and third carbons and one double bond to oxygen on the second carbon. A Amine aromatic carboxylic acid ether ketone. Which of the following list the correct functional groups found in aspartame the artificial sweetener.
It is important to be able to recognize the functional groups and the physical and chemical properties that they afford compounds. In organic chemistry the most common functional groups are carbonyls CO alcohols -OH carboxylic acids CO 2 H esters CO 2 R and amines NH 2. The carbonyl group of an.
CO stretching of aldehyde group 1720 to 1740 cm-1. More complicated chemical molecules may contain more than one functional group within their structure which can sometimes. Science Chemistry QA Library DRAW SIMPLE ORGANIC MOLECULES THAT CONTAIN THE FOLLOWING FUNCTIONAL GROUPS.
Simple molecules that contain the same functional group in their structure can be expected to react in similar ways. Amine carboxylic acid c. Which of the following are aromatic hydrocarbons.
- NH2 CH 2 NH 2 cats _ N - H ZAN - CHINHz methylamine Colts Diethylamine Aniline Benzy amine Ethere R - O - R Hge - 0. COOH is the symbol which represent the carboxylic acid functional group. CH3CHNH2CH3 I CH3CH2CH2NH2 II CH3CH2CONH2 III CH3CH2NHCH3 IV IIIIV IIIIIV IIIIIV CH3CHNH2CH3 I CH3CH2CH2NH2 II CH3CH2CONH2 III CH3CH2NHCH3 IV IIIIV IIIIIV IIIIIV.
Which of the following molecules contain the same functional groups. Which of the following molecules are aliphatic hydrocarbons. A carboxylic acid is an organic compound that contains the carboxyl functional group.
B Amine amide aromatic carboxylic acid ester. CH3CHOHCH3 A I II IV B I II III C II III IV D I III IV. Aldehydes are acyclic compounds.
Both sugars contain three carbon atoms. Carbonyl compounds all contain the carbonyl group. Compounds with C-C single bonds and C-H bonds only no other functional groups Connecting carbons can lead to large or small molecules The formula for an alkane with no rings in it must be C nH 2n2 where n is the number of carbon atoms Alkanes are saturated with hydrogen no more can be.
Functional Groups in the field of organic chemistry are the substituent atoms or groups of atoms that are attached to specific molecules. The second sugar has one double bond to oxygen on the first carbon and an OH group on both the second and third carbons. CH3CH NH2CH3 CH3CH2CH2NH2 CH3CH2CONH2 CH3CH NHCH3 I.
A functional group is a group of atoms or bonds. So functional groups may be -COOH or ketone R2CO. Aromatic ring double bond alcohol 32 Propose structures for simple molecules that contain the following functional groups.
Double bond ketone ester d. Aldehydes contain a hydroxyl group. 2 functional groupsAmino acids are organic compounds that contain amino NH3 and carboxylate CO2 functional groups along with a side chain R group specific to each amino acid.
Ketones contain a carbonyl group. Probably the most important set of heteroatomic functional groups is the set that contains carbon-oxygen double bonds. A functional group in chemistry is the part of the molecule that gives it its particular reactivity.
DRAW SIMPLE ORGANIC MOLECULES THAT CONTAIN THE. A Alcohol b Aromatic ring c Carboxylic acid d Amine e both ketone and amine f two double bonds Solutions. Of the carbonyl group with the carbon atom also being bonded to a hydroxyl OH group.
Cl na mg cl na cl. Amide double bond b. Which of the following correctly matches the molecules to the names of the functional groups.
Heteroatomic functional groups contain atoms other than carbon and hydrogen.
2 3 Functional Groups Organic Chemistry I
Comments
Post a Comment